How long can a unique atomic state live?
Atoms normally live in their ground state, where its electrons are sitting in their lowest possible orbits. But when the atoms are hit with some extra energy, their electrons are kicked into a higher energy level, orbiting further from the nucleus of the atom. That’s an excited state.
Long-lived excited states are appealing to physicists for several reasons. At JILA sophisticated optical atomic clocks need long-lived, excited strontium atoms for precise timekeeping.
Nevertheless, a single atom does not remain excited forever. How long that excited state will last depends on the species of atom, but eventually the atom shoots out the excess energy via a burst of light or collision with another atom or surface. Then the electrons decay back to their ground state.
And reaching a very long-lived state is tricky. Very long-lived states are hard to excite and typically require very sophisticated laser technologies to hold atoms in vacuum. Ideally, scientists would like to work with states that are both easily excitable and live for a long time.
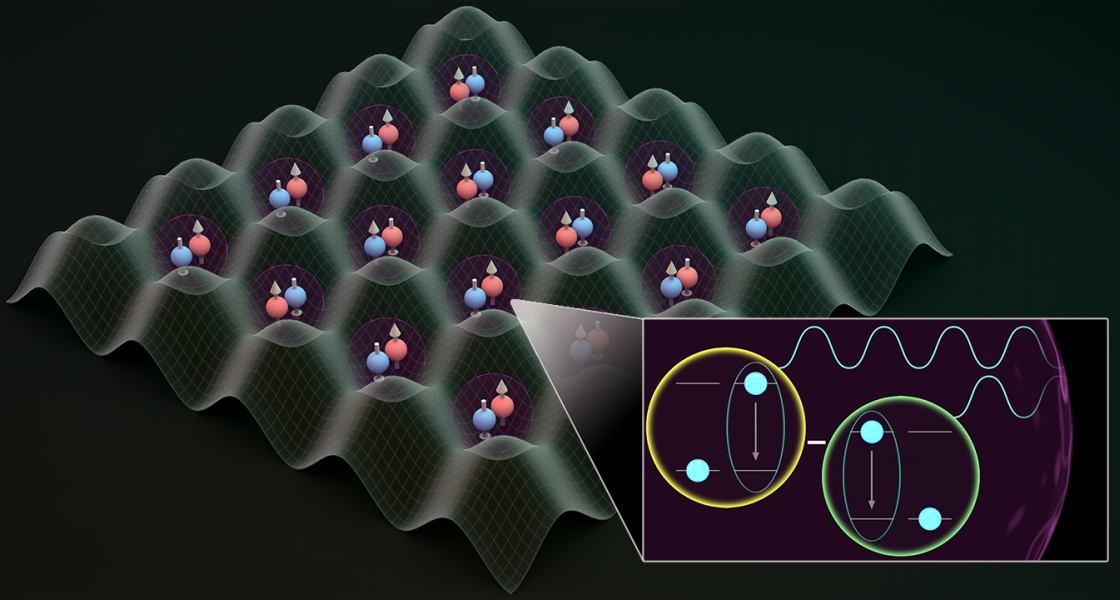
Quantum mechanics offers hope that atoms could live longer in an excited state—the creation of a dark state. “Dark states are stable and they do not decay,” said JILA Fellow Ana Maria Rey. “And there is a possibility that they live forever…this idea has caught a lot of attention in physics.”
After playing around with some material, Asier Piñeiro, a research associate in the Rey Theory Group at JILA, found a way to harness the power of the dark state.


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.